2. Pathogen - Immune cell interactions
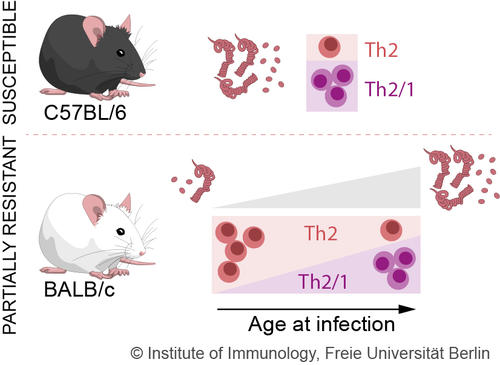
Fig. 1: Effects of ageing on the immune response against nematodes (Kapse et al. 2022)
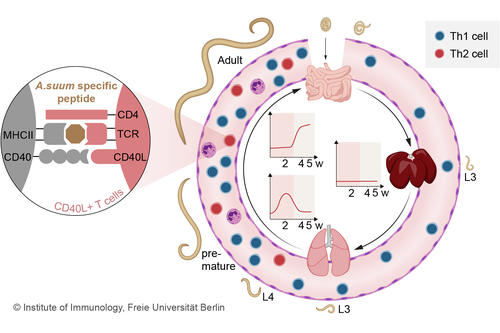
Fig. 2 T cell response during the course of an A. suum infection in the pig (Oser et al. 2024)
2.1 Th2/1 hybrid cells in nematode-infected patients and mice
In nematode infections, Th2/1 hybrid cells are induced, which we showed to stably express the transcription factors of the T helper type 1 and type 2 cells (Peine et al., 2013). Blood cells from patients infected with the threadworm Strongyloides stercoralis also showed significantly increased levels of not only GATA-3+ Th2 cells, but also GATA-3+T-bet+ cells. These Th2/1 hybrid cells are thus detectable in human infections, but express higher levels of IFN-g with very low GATA-3 expression compared to murine Th2/1 hybrids (Bock et al., 2017).
Interestingly, we also showed T cell numbers and their functions change with increasing age in response to a nematode infection. In older mice, compared to young mice, more T helper cells of type Th1 and T hybrid cells are detectable in response to the small intestinal nematode H. polygyrus (Fig. 1). In contrast, prominent T helper cell type Th2 response are found in young mice. This ageing phenomenon is associated with increased INF-g production in mice (Kapse et al., 2022).
Selected Publications
- Kapse, B.; Zhang, H.; Affinass, N.; Ebner, F.; Hartmann, S.; Rausch, S. (2022): Age-dependent rise in IFN-γ competence undermines effective type 2 responses to nematode infection. Mucosal Immunol; 15(6), S. 1270–1282
- Zhang, H.; Bednář, L.; Heitlinger, E.; Hartmann, S.; Rausch, S. (2022): Whip- and pinworm infections elicit contrasting effector and distinct regulatory responses in wild house mice. Int J Para; 52(8), S. 519–524
- Affinass, N.; Zhang, H.; Löhning, M.; Hartmann, S.; Rausch, S. (2018):Manipulation of the balance between Th2 and Th2/1 hybrid cells affects parasite nematode fitness in mice. Eur J Immunol; 48(12), S. 1958–1964
- Bock, C.N., S. Babu, M. Breloer, A. Rajamanickam, Y. Bhootra, M.-L. Brunn, A. A. Kühl, R. Merle, M. Löhning, S. Hartmann, S. Rausch (2017): Th2/1 hybrid cells occurring in murine and human strongyloidiasis share effector functions of Th1 cells, Frontiers in Cellular and Infection Microbiology, doi: 10.3389/fcimb.2017.00261.
- Peine, M., S. Rausch, C. Helmstetter, A. Fröhlich, A. N. Hegazy, A. A. Kühl, C. G. Grevelding, T. Höfer, S. Hartmann, and M. Loehning (2013): Stable T-bet+GATA-3+ Th1/Th2 hybrid cells arise in vivo, can develop directly from naïve precursors, and limit immunopathologic inflammation. PLoS Biology, 11:e1001633.
Third-party funding: DFG GRK 2046: S. Rausch Project B5.
2.2 Tools and T cell responses in pigs
In order to characterize T cell populations in pigs in response to infections, we have established the detection of lineage-specific T cell transcription factors and of antigen-specific T cells in pigs (Ebner et al., 2014, Schmidt et al., 2020). Using the marker CD154, pathogen-specific T cells can be analyzed during infection. A. suum-infected pigs showed a local, transient Th2 response in the lung and an accumulation of Th2 cells in the intestine after body migration of the larvae (Fig. 2), which was accompanied by a systemic Th1 expansion (Oser et al. 2024). The porcine model allows the characterization of pathogen-specific T cells of human-relevant infections and their distribution in the organs of the infected individual.
Selected Publications
- Oser, L., Midha, A., Schlosser-Brandenburg, J., Rausch, S., Mugo, R. M.; Kundik, A., Elizalde-Velázquez, L. E., Adjah, J., Musimbi, Z. D., Klopfleisch, R.; Helm, C. S., von Samson-Himmelstjerna, G., Hartmann, S., Ebner, F. (2024): Ascaris suum infection in juvenile pigs elicits a local Th2 response in a setting of ongoing Th1 expansion, Front Immunol; doi: 10.3389/fimmu.2024.1396446
- Schlosser-Brandenburg, J.; Ebner, F.; Klopfleisch, R.; Kühl, A. A.; Zentek, J.; Pieper, R.; Hartmann, S. (2021): Influence of nutrition and maternal bonding on postnatal lung development in the newborn pig. Front Immunol; 12, S. Article 734153
- Schmidt, S.; Ebner, F.; Rosen, K.; Kniemeyer, O.; Brakhage, A. A.; Löffler, J.; Seif, M.; Springer, J.; Schlosser, J.; Scharek-Tedin, L.; Scheffold, A.; Bacher, P.; Kühl, A. A.; Rösler, U.; Hartmann, S. (2020):The domestic pig as human‐relevant large animal model to study adaptive anti‐fungal immune responses against airborne Aspergillus fumigatus. Eur J Immunol; 50(11), S. 1712–1728
- Ebner, F.; Morrison, E.; Bertazzon, M.; Midha, A.; Hartmann, S.; Freund, C.; Álvaro-Benito, M. (2020): CD4+ Th immunogenicity of the Ascaris spp. secreted products. Vaccine; 5, S. Article number: 25
- Ebner, F., P. Schwiertz, S. Steinfelder, R. Pieper, J. Zentek, N. Schütze, C. G. Baums, G. Alber, P. Geldhof, S. Hartmann. 2017. Pathogen-reactive T helper cell analysis in the pig, Frontiers in Immunology, doi: 10.3389/fimmu.2017.00565.
Third-party funding: DFG GRK 2046: F. Ebner Project C9.
2.3 Regulation of antigen-presenting cells in nematode infections
Nematodes induce regulatory monocytes/macrophages, which are phagocytes and antigen-presenting cells. We demonstrated regulatory macrophages in mice treated with nematode antigen (Klotz et al., 2011) and in the blood of filarial-infected patients in South India (O'Regan et al., 2014). These regulatory macrophages influence independent inflammatory responses. Administration of nematode-induced regulatory macrophages in independent inflammatory events suppressed the inflammation and induced upregulation of the anti-inflammatory cytokine IL-10 by T helper cells (Ziegler et al., 2015). Thus, nematode-induced regulatory macrophages protect the host from nematode-induced pathology, but also from independent mucosal inflammation (Steinfelder et al., 2016).
Further, the intestinal porcine roundworm Ascaris suum utilized their secreted/excreted (ES) products to modulate the key antigen-presenting cell, the dendritic cell. Nematode ES induced a change in important dendritic cell surface markers and a suppression of the pro-inflammatory cytokine production IL-12 and TNF-a (Hamid et al., 2022).
Selected Publications
- Hamid, B.; Ebner, F.; Bechtold, L.; Kundik, A.; Rausch, S.; Hartmann, S. (2022):Ascaris suum excretory/secretory products differentially modulate porcine dendritic cell subsets.Front Immunol; 13, S. Artikel 1012717
- Hamid, B.; Schlosser-Brandenburg, J.; Bechtold, L.; Ebner, F.; Rausch, S.; Hartmann, S. (2021): Early immune initiation by porcine cells following Toxoplasma gondii infection versus TLR ligation. Microorganisms; 9(9), S. Artikel 1828
- Schlosser-Brandenburg, J.; Ebner, F.; Klopfleisch, R.; Kühl, A. A.; Zentek, J.; Pieper, R.; Hartmann, S. (2021): Influence of nutrition and maternal bonding on postnatal lung development in the newborn pig. Front Immunol; 12, S. Article 734153
- Strandmark, J.; Steinfelder, S.; Berek, C.; Kühl, A.; Rausch, S.; Hartmann, S. (2016): Eosinophils are required to suppress Th2 responses in Peyer’s patches during intestinal infection by nematodes. Mucosal Immunol; 10, S. 661–672
- Steinfelder, S., N.L. O’Regan, S. Hartmann (2016): Diplomatic Assistance: Can Helminth modulated macrophages act as treatment for inflammatory disease, PLoS Pathogens, DOI:10.1371
- Ziegler, T., S. Rausch, S. Steinfelder, C. Klotz, M.R. Hepworth, A. Kühl, P-C- Burda, R. Lucius, S. Hartmann (2015): A novel regulatory macrophage (Mreg) induced by a helminth molecule instructs IL-10 in CD4+ T-cells and protects against mucosal inflammation, J Immunology, 194:1555-1564.
Third-party funding: DFG GRK 2046: S. Hartmann Project B4.