AG Bröer
Arbeitsgruppe Prof. Dr. Sonja Bröer

I am a veterinarian (graduated in 2010 from TiHo Hannover) as well as an internationally well-connected scientist and enthusiastic teacher. I hope to mentor in programs that help students succeed in their careers (also outside of academic or industry biomedical sciences). Feel free to set up an appointment and stop by my office!
Our research in neurosciences and neuropharmacology is inspired by multidisciplinary approaches evaluating the role of the immune system and de- and regeneration of neurons in diseases of the central nervous system. We focus on epilepsy as one of the most common chronic neurological diseases. Seizures can be triggered by various causes, such as infections and intoxications of the central nervous system, genetic predispositions, stroke, and head trauma. Despite the multifaceted etiology current treatment approaches mainly aim to suppress seizures symptomatically and do not treat the underlying cause of disease development and progression. My work is oriented towards identifying new pharmacological targets that could alter processes that are contributing to seizure generation and brain pathology, such as stem cell differentiation (e.g., neurogenesis), neurodegeneration, and inflammation.
Here you can see a short clip about our research in regenerative medicine (in German, Video Stammzellforschung).
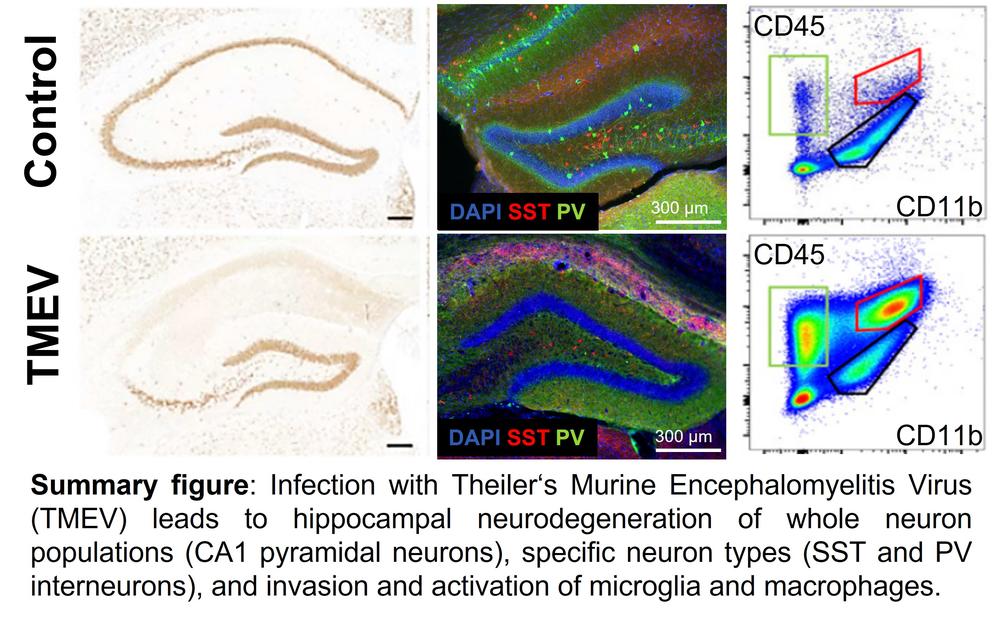
Especially in an inflammatory state the delicate balance of immune cells that are activated or infiltrate the brain after an infection seems to be key to seizure development and neurological damage. I am particularly interested in the innate immune response. Monocytes adopt different functions depending on their activation cues; they might help promote tissue repair, but their ability to fight infected cells can be detrimental to the healing of affected tissue. I am working with a model mimicking the infectious etiology of epilepsy, in which we could show that the death of CA1 neurons in the hippocampus, as well as the activation of microglia and the infiltration of peripheral macrophages, are biomarkers of seizure development. Together with my previous team in the group of Prof. Dr. Löscher at the TiHo in Hannover, as well as a very successful cooperation with the Dept. of Pathology, and the TwinCore Helmholtz Center for Infection Research, I discovered that as early as two days post viral infection macrophages migrate into the brain and simultaneously resident microglia are activated. Further investigation on macrophage reporter mice allowed a more precise differentiation of the involved immune cells and their surface antigens and showed that a subpopulation of microglia was no longer distinguishable from migrating macrophages after activation. By using additional genetically modified mice (Ccr2-/- and Cx3cr1-/-) we were able to further elucidate the significance of the individual cell populations for the onset of seizures: Both microglial activation and macrophage invasion independently lead to acute seizures. However, the associated neurodegeneration in the hippocampus could be significantly reduced by switching off one of the cell populations. The mechanisms of seizure formation and neurodegeneration after viral encephalitis appear to be more complex than initially thought and require further studies to be fully understood.
Apart from disease mechanisms, I have a strong interest in researching new translational therapeutic strategies for neurological diseases. In the past few years, I have worked as a leading scientist in a Silicon Valley-based biotechnology start-up, Neurona Therapeutics. The company is developing a stem cell-based therapy for neurodegenerative diseases, including epilepsy. I have gained in-depth knowledge about cell-based therapies and building a preclinical program on its successful route toward clinical approval, which I will integrate into my research and teaching endeavors at the FU.
Selected publications:
Bershteyn M*, Bröer S*, Parekh M*, Maury Y*, […], Nicholas CR (2023).
Human pallial MGE-type GABAergic interneuron cell therapy for chronic focal epilepsy.
Cell Stem Cell. 30(10):1331-1350.e11.
*contributed equally
Batot G, Metcalf CS, Bell LA, Pauletti A, Wilcox KS, Bröer S (2022).
A Model for Epilepsy of Infectious Etiology using Theiler's Murine Encephalomyelitis Virus.
J Vis Exp. 184: e63673.
Henke C, Töllner K, van Dijk RM, Miljanovic N, Cordes T, Twele F, Bröer S, […], Löscher W (2020).
Disruption of the sodium-dependent citrate transporter SLC13A5 in mice causes alterations in brain citrate levels and neuronal network excitability in the hippocampus.
Neurobiol Dis. 143:105018.
Debski KJ, Ceglia N, Ghestem A, Ivanov AI, Brancati GE, Bröer S, […], Bernard C (2020).
The circadian dynamics of the hippocampal transcriptome and proteome are altered in experimental temporal lobe epilepsy.
Sci Adv. 6(41):eaat5979.
Käufer C*, Chhatbar C*, Bröer S*, Waltl I, Ghitab L, Gerhauser I, Kalinke U, Löscher W (2018).
Chemokine receptors CCR2 and CX3CR1 regulate viral encephalitis-induced hippocampal damage but not seizures.
Proc Natl Acad Sci USA 115:E8929–E8938.
*contributed equally
Backofen-Wehrhahn B, Gey L, Bröer S, Petersen B, [….], Gernert M (2018).
Anticonvulsant effects after grafting of rat, porcine, and human mesencephalic neural progenitor cells into the rat subthalamic nucleus.
Exp Neurol 310:70-83.
Waltl I, Käufer C, Bröer S, Chhatbar C, Ghita L, Gerhauser I, Anjum M, Kalinke U, Löscher W (2018).
Macrophage depletion by liposome-encapsulated clodronate suppresses seizures but not hippocampal damage after acute viral encephalitis.
Neurobiol Dis 110:192-205.
Anjum SMM, Käufer C, Hopfengärtner R, Waltl I, Bröer S, Löscher W (2018).
Automated quantification of EEG spikes and spike clusters as a new readout in Theiler's virus mouse model of encephalitis-induced epilepsy.
Epilepsy Behav. 88:189-204.
All publications:
https://www.ncbi.nlm.nih.gov/sites/myncbi/1h57IXY9uzu5e/collections/59872833/public/